SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Sulfor dioxide: Lewis dot structure for SO2 (video) | Khan Academy Chemistry library Course: Chemistry library > Unit 9 Lesson 4: Dot structures and molecular geometry Resonance and dot structures Formal charge Formal charge and dot structures Worked example: Using formal charges to evaluate nonequivalent resonance structures
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of Molecules
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

How to draw SO2 Lewis Structure? Science Education and Tutorials
This video shows how to draw Lewis dot structure of SO2 - how to draw a coordinated bond by using Chemsketch

SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram Techiescientist
A dot structure is any representation of atoms/molecules using dots for electrons. And a Lewis diagram (or Lewis structure or Lewis dot structure) is a type of dot structure created by the chemist Gilbert N. Lewis which is most commonly used in chemistry nowadays. There's a slight difference, but they effectively mean the same thing.

SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
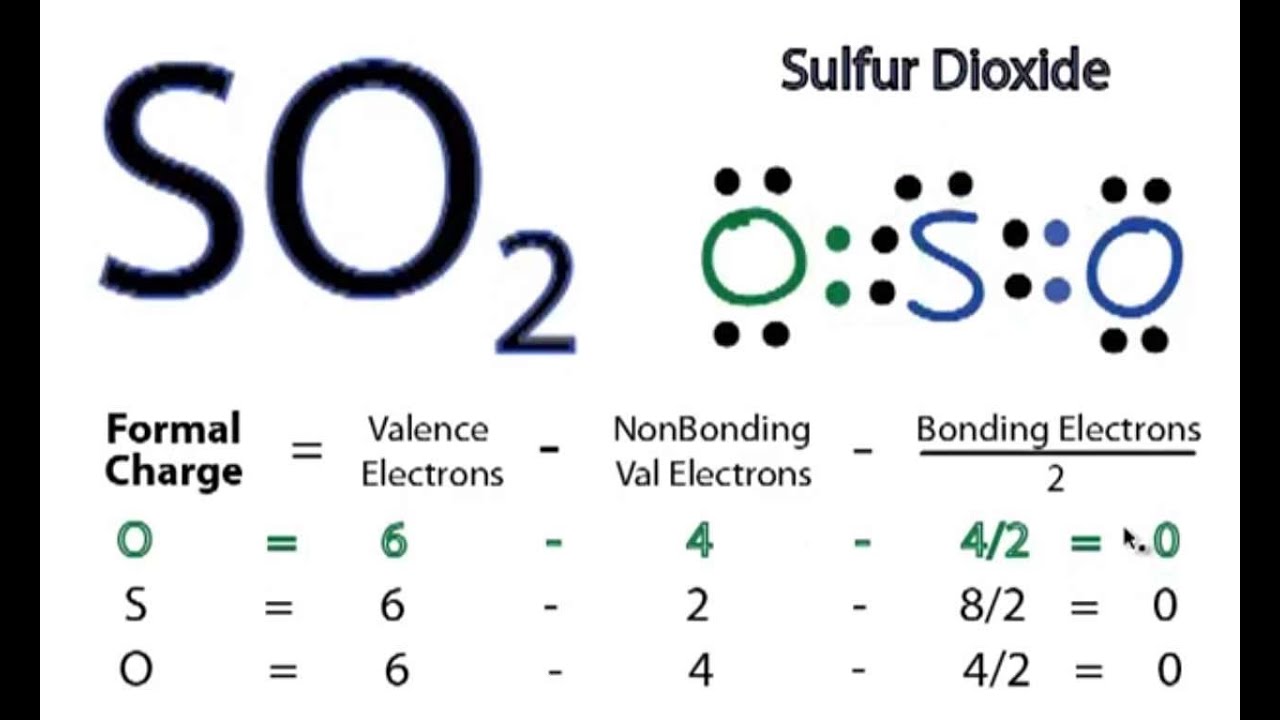
Exercise Draw Lewis dot structures for CH 4, NH 3, HF, OF 2, F 2, O 2, N 2, Cl − and some compounds you know. Formal Charge The formal charge on any atom in a Lewis structure is a number assigned to it according to the number of valence electrons of the atom and the number of electrons around it.

How to draw SO2 Lewis Structure? Science Education and Tutorials
A Lewis structure is a way to show the shape of a molecule. Dots show where electrons are around the atoms, and lines or pairs of dots show where covalent bonds connect the atoms. By drawing a Lewis dot structure, you can find the lone electron pairs in molecules, which helps you figure out how chemical bonds form.

SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
This chemistry video tutorial explains how to draw the lewis structure of SO2 also known as Sulfur Dioxide. It discusses the molecular geometry, bond angle, hybridization and formal charges.
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of Molecules
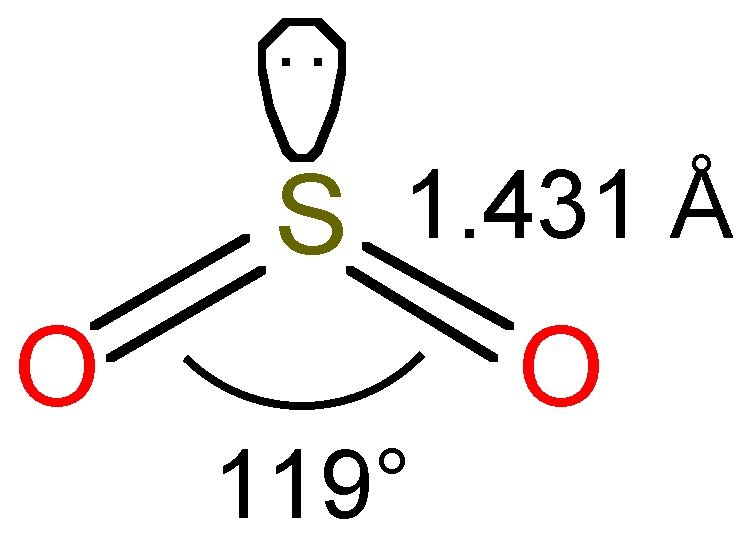
A step-by-step explanation of how to draw the SO2 Lewis Structure (Sulfur Dioxide) Note: From an experimental view (using x-ray crystallography or someth.
SO2 Lewis Structure How to Draw the Lewis Structure for SO2 (Sulfur Dioxide) YouTube
Lewis Structure of SO2 (sulfur dioxide) - YouTube 0:00 / 4:59 Lewis Structure of SO2 (sulfur dioxide) chemistNATE 259K subscribers Subscribe 6.6K Share 902K views 9 years ago Lewis.
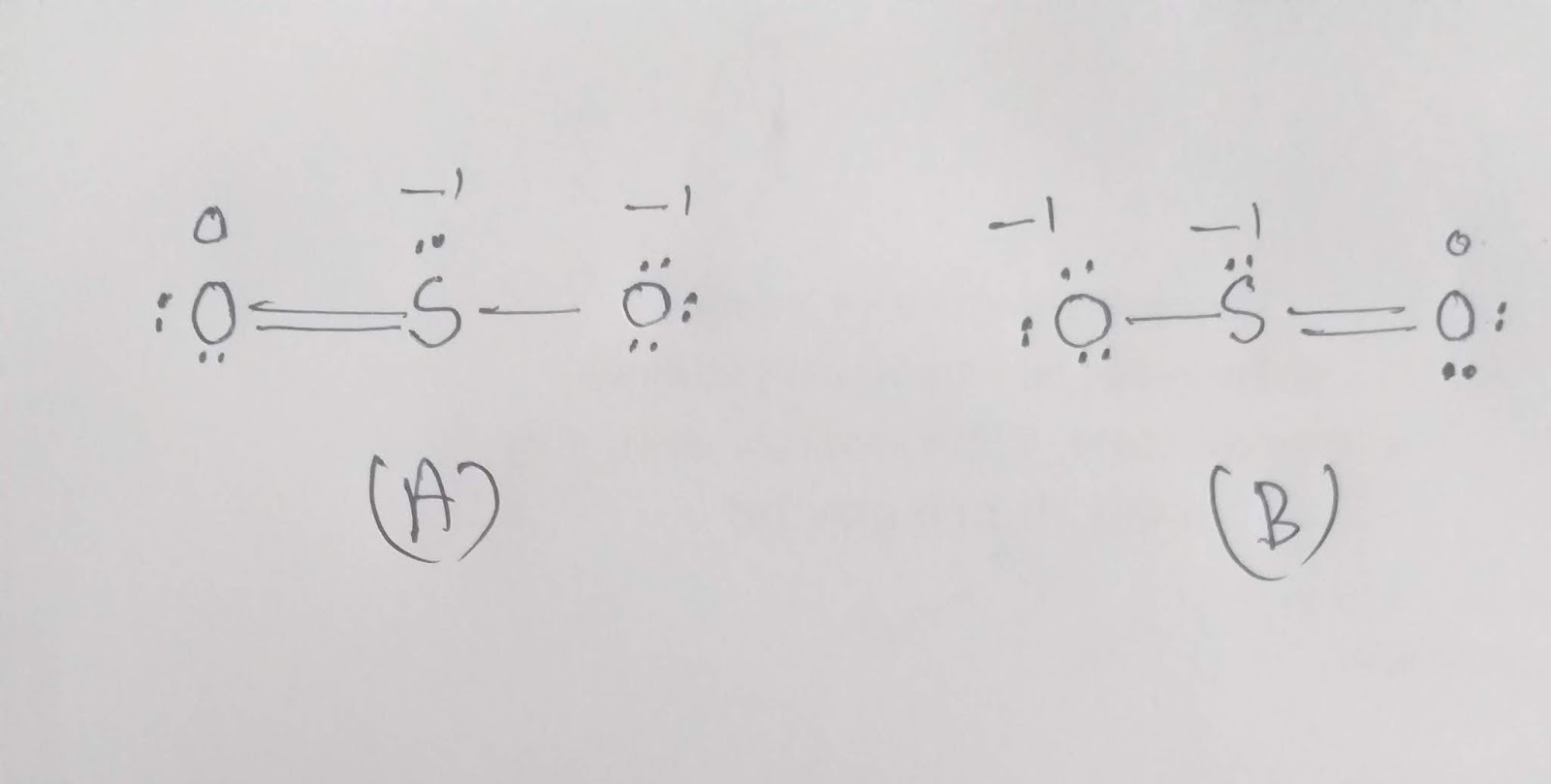
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
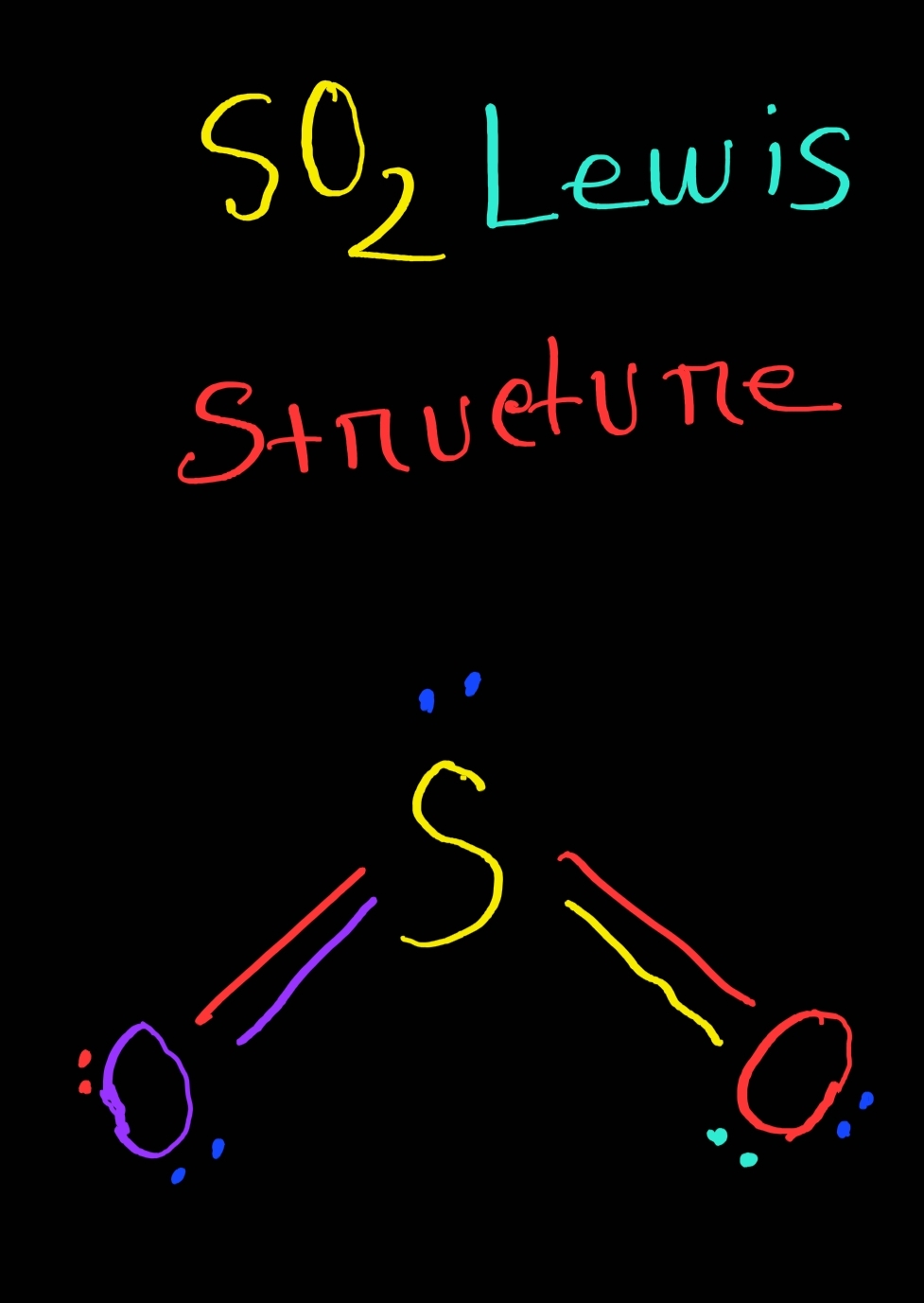
Lewis structure is the distribution of the electrons around the atoms of a compound. This structure helps us to know about the kind of bonds and the number of bonds that form the compound. Now let's walk through the method of drawing lewis structure:

SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
6 Steps to Draw the Lewis Structure of SO2 Step #1: Calculate the total number of valence electrons Here, the given molecule is SO2 (sulfur dioxide). In order to draw the lewis structure of SO2, first of all you have to find the total number of valence electrons present in the SO2 molecule.
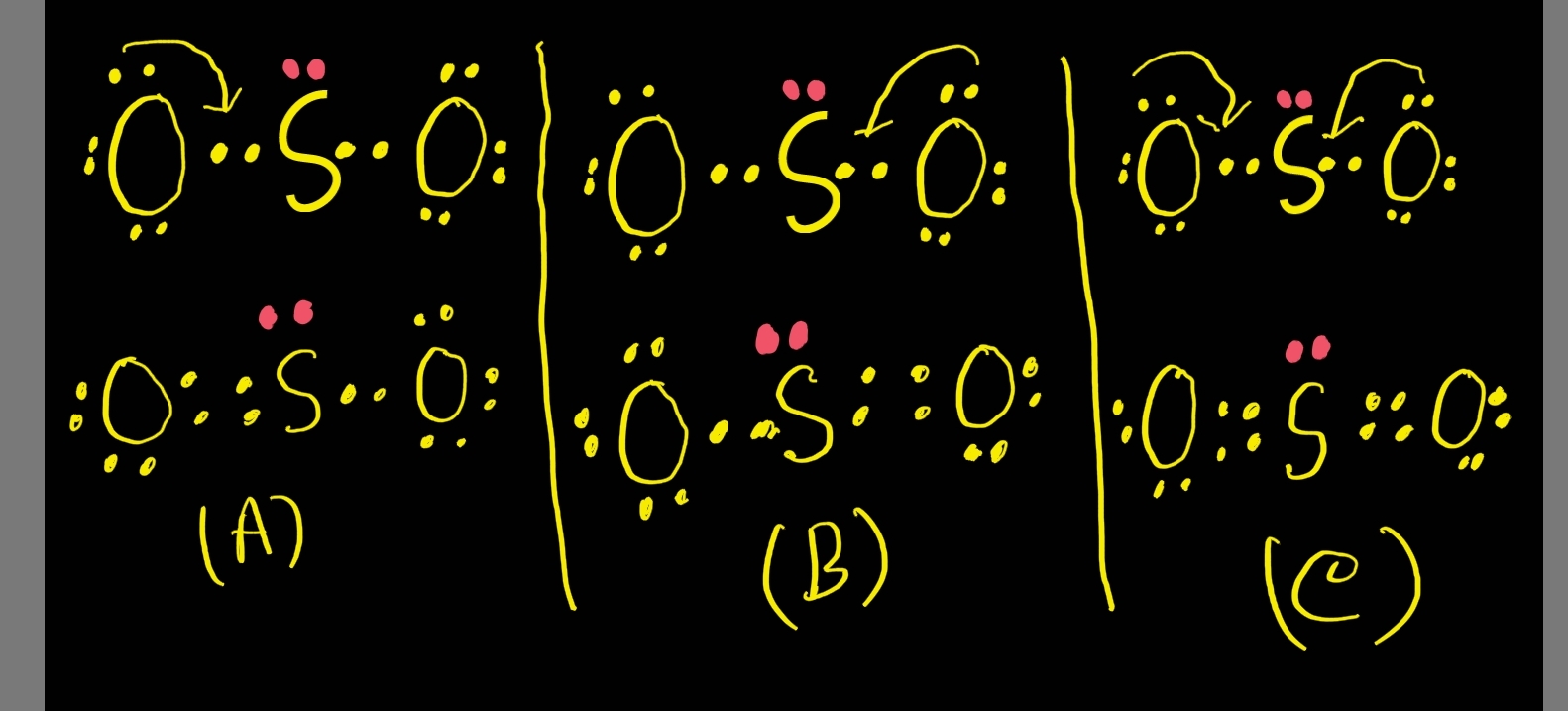
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
To sketch the SO2 Lewis structure by following these instructions: Step-1: SO2 Lewis dot Structure by counting valence electrons on the sulfur atom. Step-2: Lewis Structure of SO2 for counting valence electrons around the terminal oxygen atoms. Step-3: Lewis dot Structure for SO2 generated from step-1 and step-2.
Resonance Structures Easy Hard Science
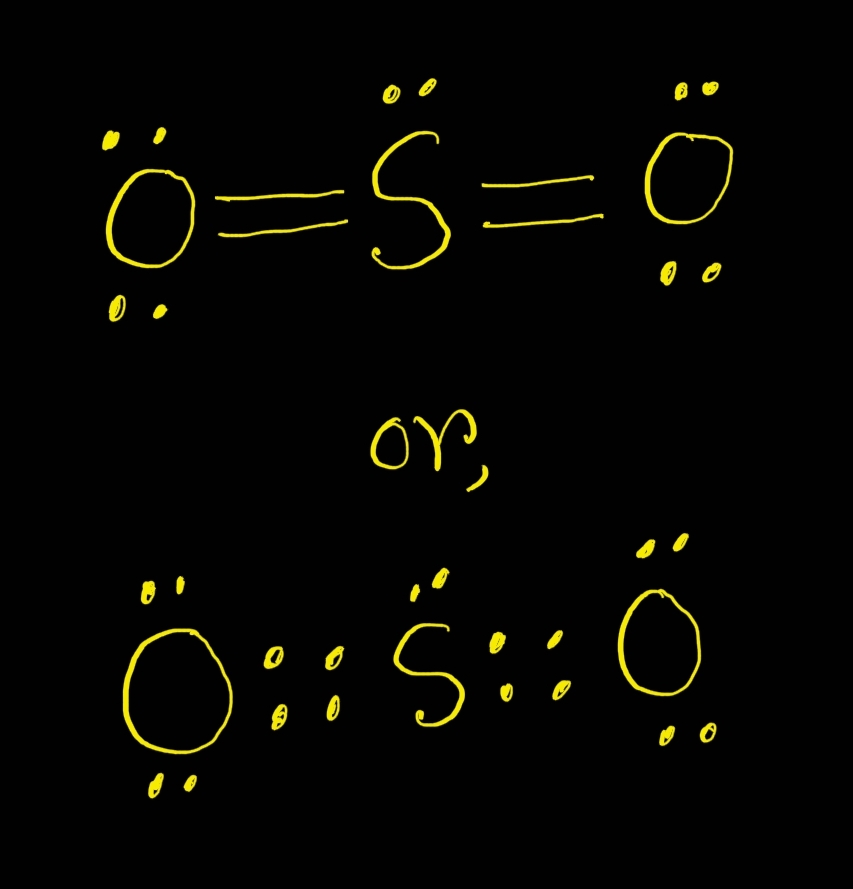
What is the structure of SO 2? I have seen two different ways the Lewis Structure is written: The formal charges of the SO 2 with the single bond and a double bond is larger than the SO 2 with two double bonds. So I would assume that the one with two double bonds is the correct structure.
SO2 Lewis Structure ,Valence Electrons ,Formal Charge Lewis Structure for SO2 (Sulfur Dioxide)
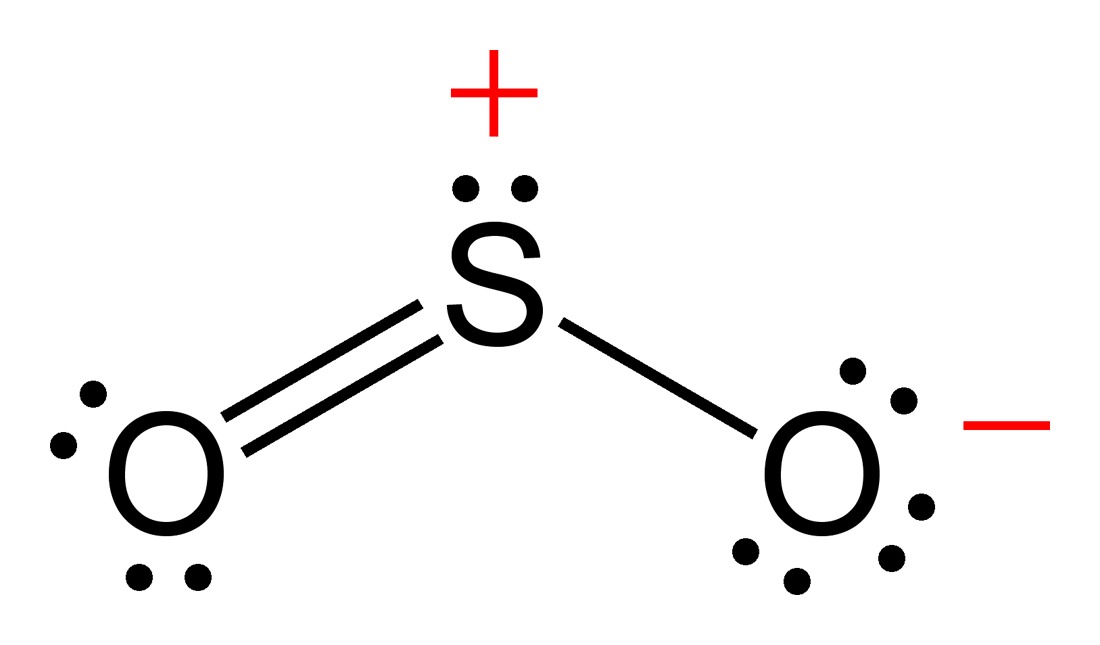
Drawing the Lewis Structure for SO 2. The Lewis structure for SO 2 requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 2 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons. You'll want to calculate the formal charges on each atom to.
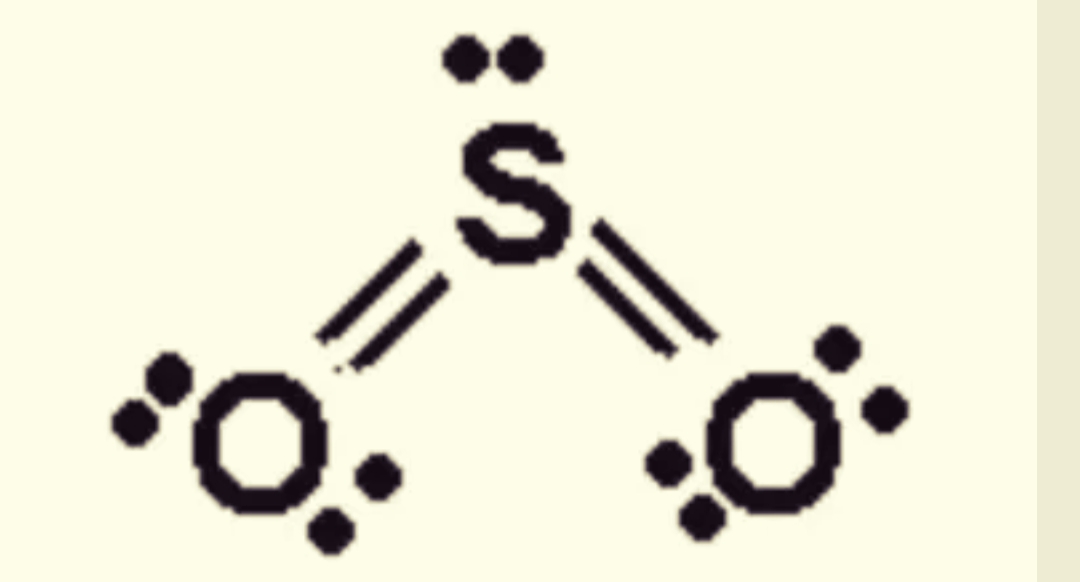
SO2 Lewis Structure ,Valence Electrons ,Formal Charge Lewis Structure for SO2 (Sulfur Dioxide)
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair of electrons: A single shared pair of electrons is called a single bond.
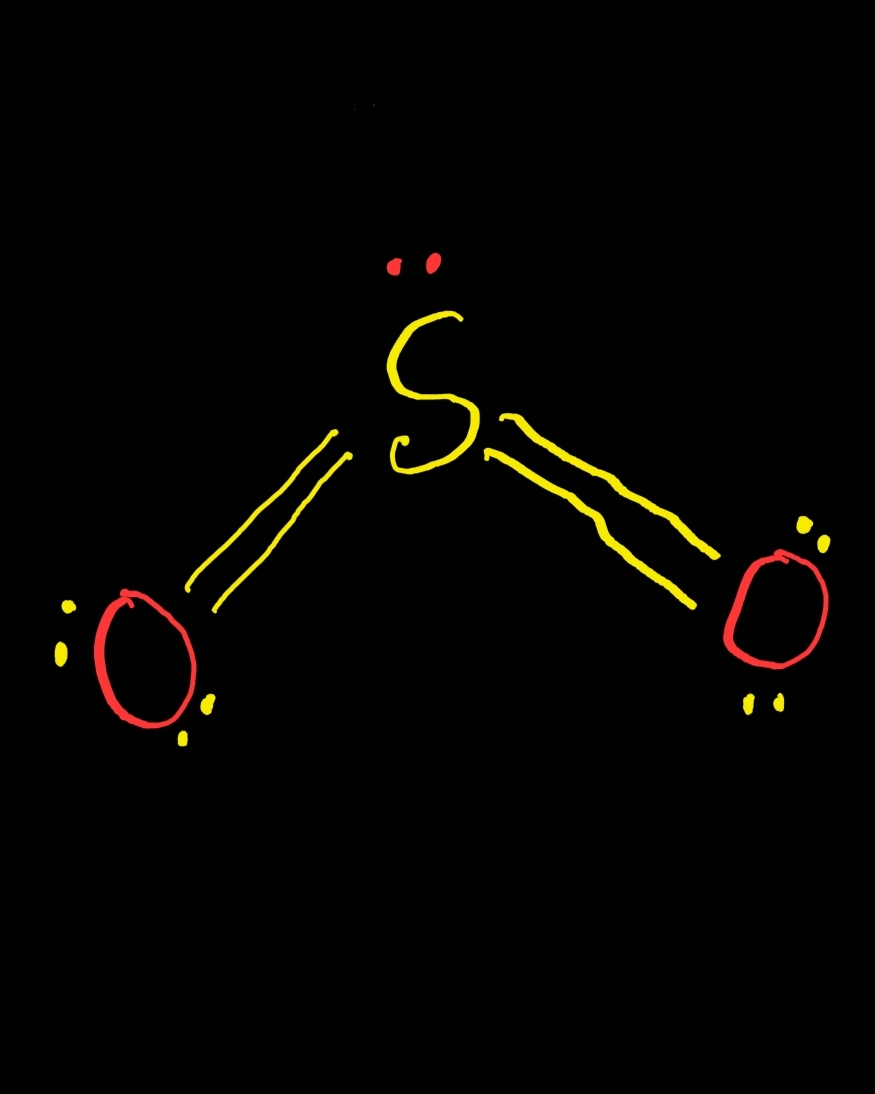
SO2 Lewis Structure ,Valence Electrons ,Formal Charge Lewis Structure for SO2 (Sulfur Dioxide)
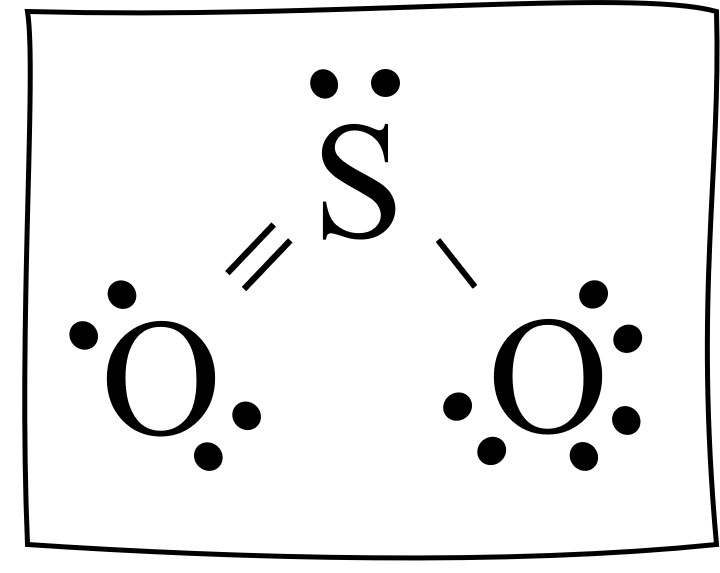
Subtract step 1 total from step 2. 24-18=6e-. Step 4: Find number of bonds by diving the number in step 3 by 2 (because each bond is made of 2 e-) 6e-/2= 3 bond pairs. Step 5: Find the number of nonbonding (lone pairs) e-. Subtract step 3 number from step 1. 18-6= 12e-=6 lone pairs. Now, use the information from step 4 and 5 to draw the Lewis.